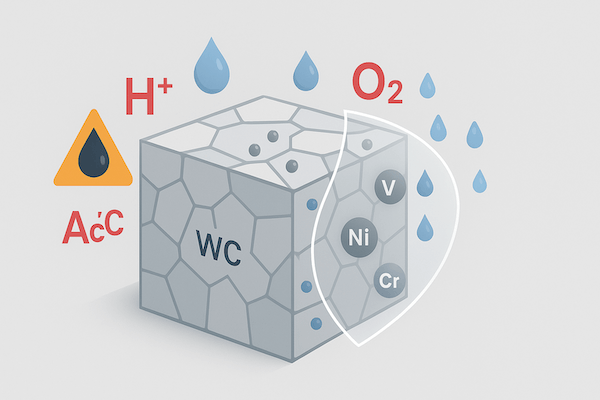
Tungsten carbide tools are widely recognized for their exceptional hardness and wear resistance—but there’s another property that’s just as vital for long-term performance: chemical stability. Whether operating in corrosive environments, exposed to coolants, or enduring high-temperature reactions, carbide tools must resist chemical degradation to maintain their functionality.
This article explains what chemical stability means, why it matters in tungsten carbide tools, and how it’s engineered into modern tool materials.
What Is Chemical Stability?
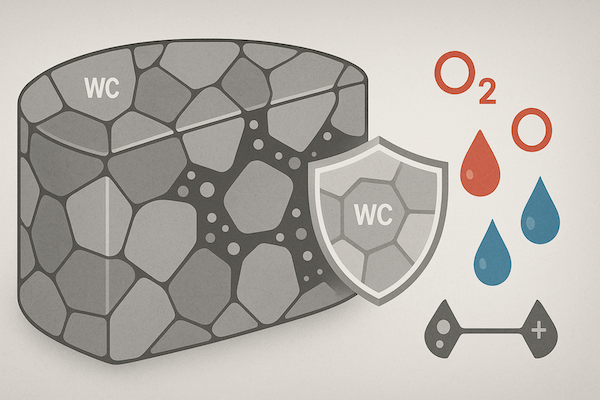
Chemical stability refers to a material’s ability to resist chemical reactions with its environment—especially oxidation, corrosion, or reactions with acids, alkalis, and gases. For cutting and wear-resistant tools, chemical stability is essential to preserve structural integrity and tool life under thermal, mechanical, or corrosive stress.
In tungsten carbide tools, this stability depends on:
The inertness of the WC ceramic phase
The resistance of the binder metal (usually cobalt or nickel)
The presence of protective alloying elements like chromium, vanadium, or molybdenum
Why Is Chemical Stability Important for Carbide Tools?
Tungsten carbide tools are used in industries where they are exposed to harsh working conditions, such as:
High-speed machining with coolant exposure
Oil & gas drilling in corrosive formations
Die and mold work in chemically aggressive environments
Food and pharmaceutical processing requiring corrosion resistance
Without sufficient chemical stability, tools can experience:
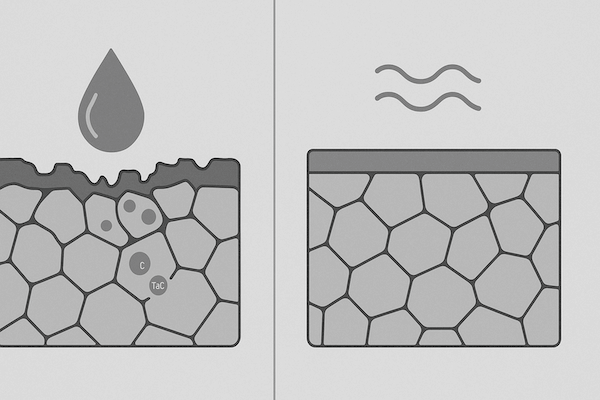
Cobalt leaching from binder phase (especially in wet or acidic conditions)
Oxidation of carbide particles at high temperatures
Loss of hardness and strength, leading to early failure
Surface pitting and material erosion
Key Factors Affecting Chemical Stability in WC Tools
Several material and design choices determine how chemically stable a tungsten carbide tool will be:
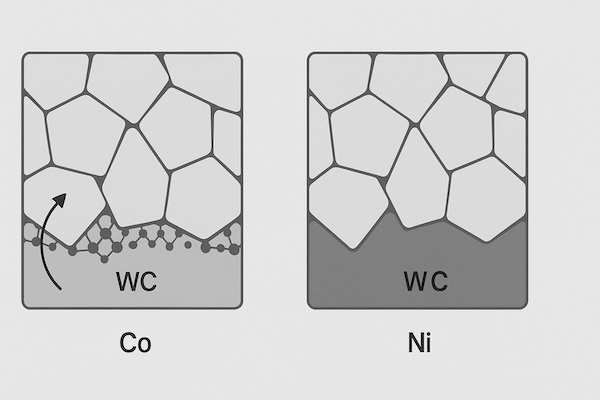
🔹 Binder Phase Composition
Cobalt is chemically reactive in certain environments. Replacing or modifying the binder with nickel or Co-Ni alloys improves corrosion resistance.
🔹 Alloying Additives
Elements like Cr (chromium), Mo (molybdenum), and V (vanadium) form stable carbides that help protect against chemical attack and oxidation.
🔹 Grain Structure
Finer, uniform grain structures reduce exposed surface area and resist intergranular corrosion more effectively.
🔹 Surface Coatings
Applying PVD or CVD coatings (e.g., TiN, TiAlN, CrN) creates a chemically inert barrier that significantly increases tool lifespan in aggressive environments.
Application Examples
| Industry | Challenge | Role of Chemical Stability |
|---|---|---|
| Aerospace | High-temp oxidation | WC+Cr/Co tools resist degradation at 700°C+ |
| Oil & Gas | Acidic well fluids | Mo- or Ni-alloyed carbides prevent cobalt leaching |
| Mold & Die | EDM and coolants | Stable binders resist corrosion and pitting |
| Medical/Pharma | Chemical washdown | Corrosion-resistant carbide tools maintain hygiene |
Conclusion
Chemical stability isn’t just a material science buzzword—it’s a defining feature of high-performance tungsten carbide tools. Whether facing high heat, caustic fluids, or chemical abrasion, tools with engineered chemical stability offer longer life, fewer failures, and lower operational costs.
By controlling composition, microstructure, and surface protection, manufacturers ensure that carbide tools meet the demands of the most chemically aggressive environments—without sacrificing performance.
